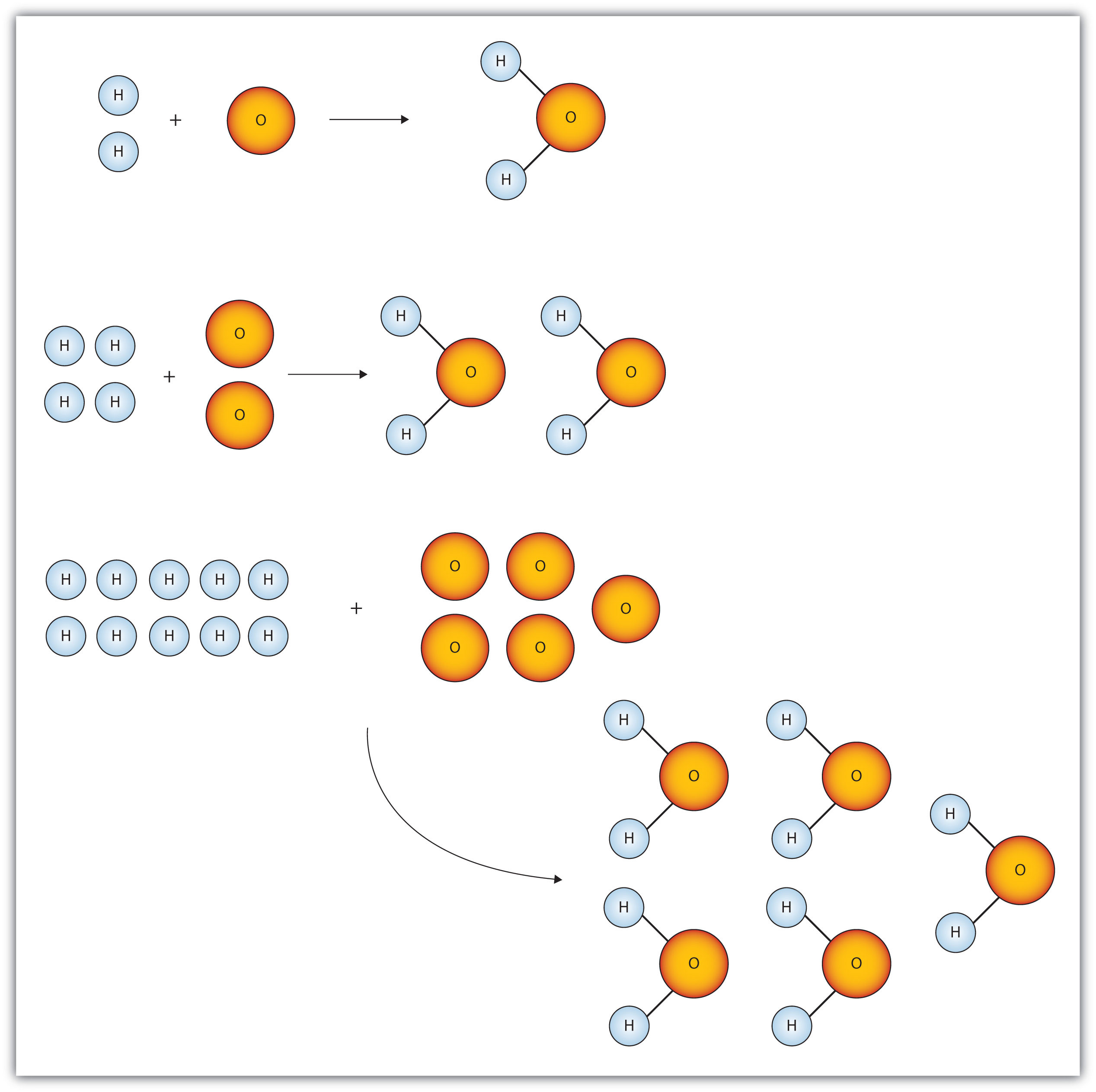
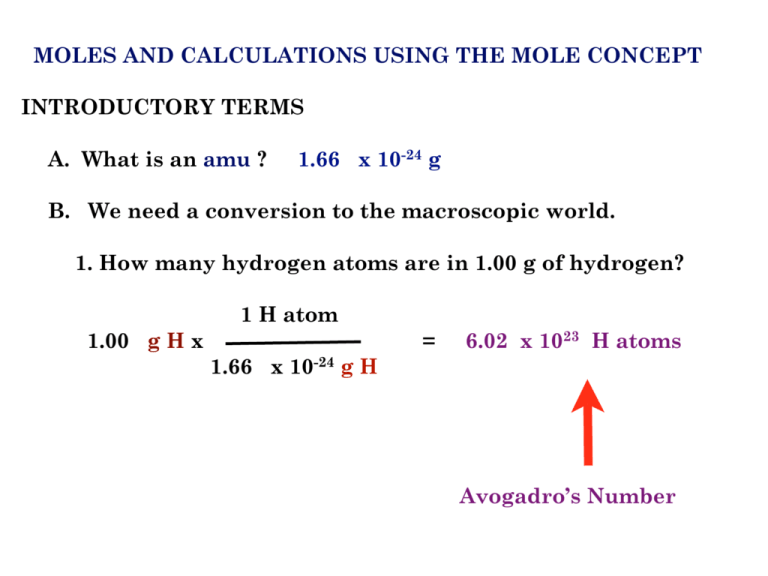
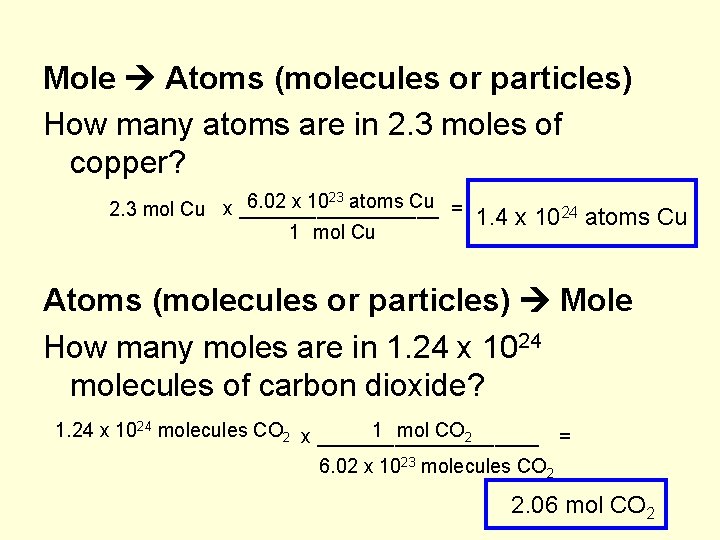
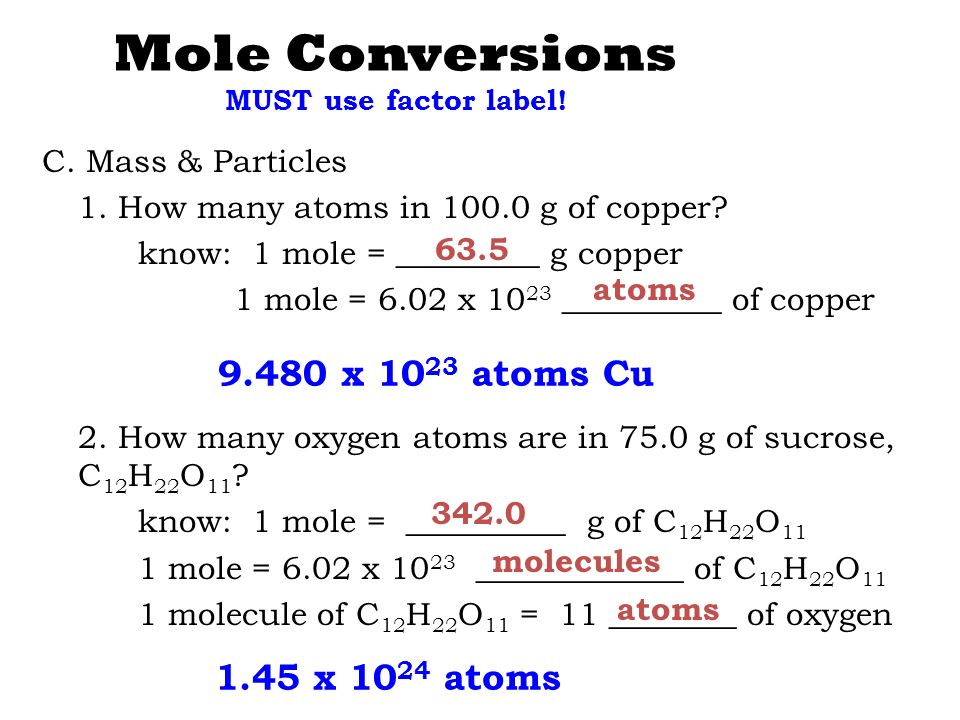
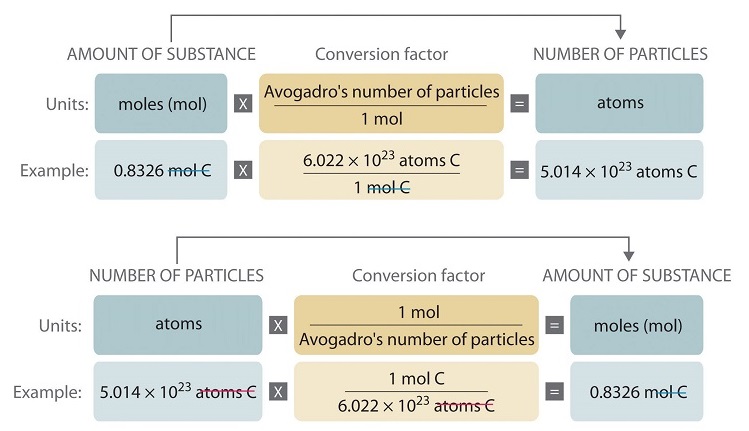
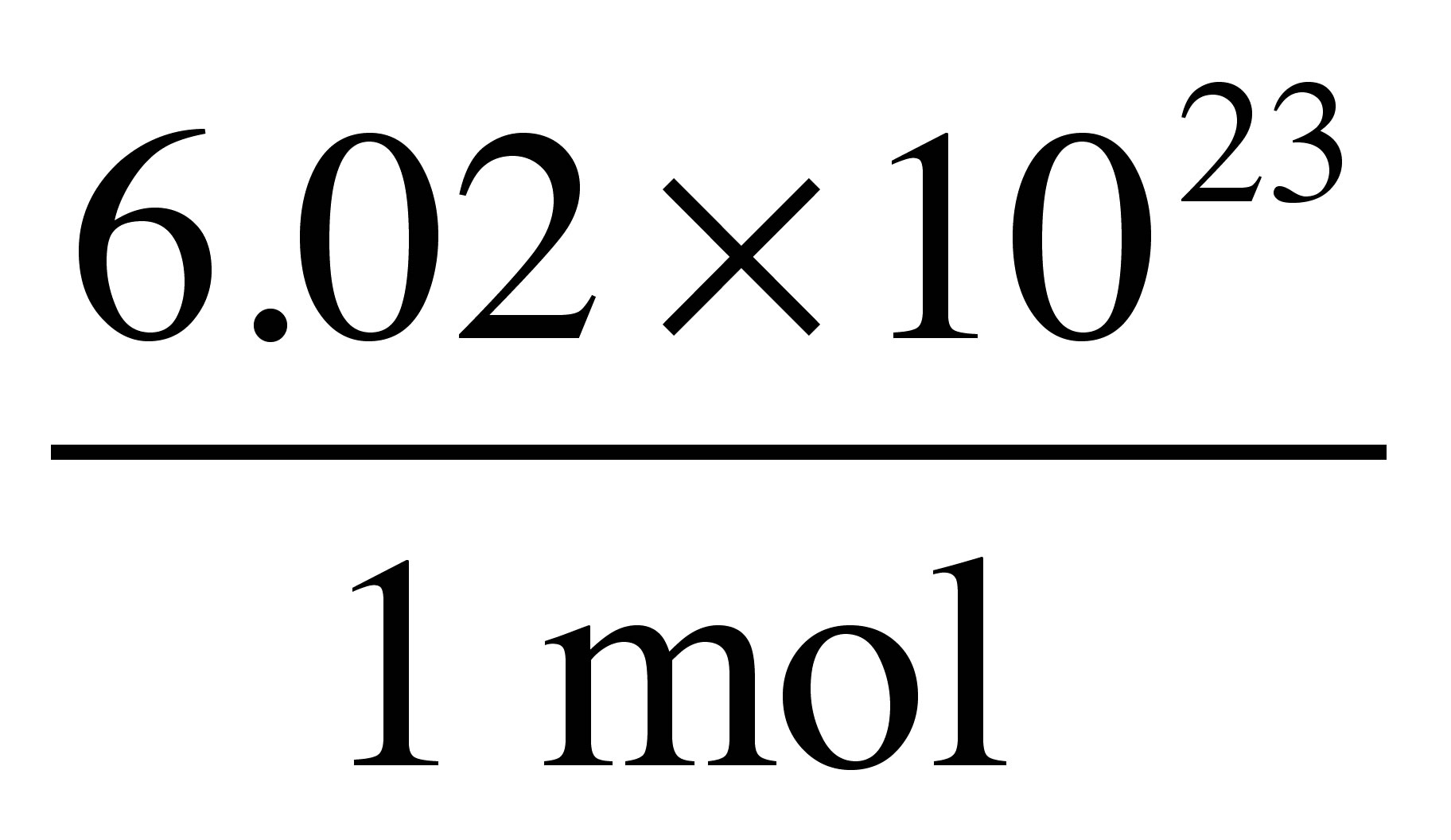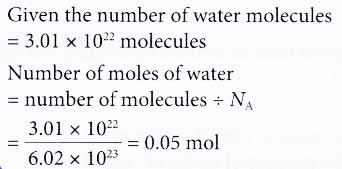The number of atoms present in one mole of an element is equal to Avogadro number. Which of the following element contains the greatest number of atoms ?

The Mole Standards 1 dozen = 1 gross = 1 ream = 1 mole = x There are exactly 12 grams of carbon-12 in one mole of carbon ppt download